Alzheimer’s disease is a clinical diagnosis, and there is, as of yet, no single, simple diagnostic test.
The diagnostic tests that exist can be invasive, expensive, and time-consuming.
Developing a simple, early diagnostic test could help to target the use of new Alzheimer’s drugs to people in the early stages of the condition.
An international team has found a blood test that detects a particular form of tau is effective at detecting changes in amyloid and tau in the brain.
A blood test that screens for Alzheimer’s disease shows promise in detecting changes in amyloid beta and tau protein levels in the brain years before symptoms emerge, according to a new study published in.
Despite Alzheimer’s disease affectingworldwide, there is still no reliable and simple way to diagnose the condition.
This has led to a situation in the United States where less than half of people with dementia have been diagnosed by a physician, according to the. Doctors normally make a diagnosis by taking a history of symptoms and excluding the possibility of other types of dementia, such as vascular dementia, using scans, or cerebrospinal fluid tests. These are all time-consuming, expensive, and potentially invasive.
Lack of accuracy in testing means detecting the disease in its early stages is difficult. A result of this is that the few treatment options that are available for Alzheimer’s disease can’t be started early when they might be the most effective, and it is also hard to design trials to determine if certain drugs would be effective if started in the early stages of the disease.
Why early detection of Alzheimer’s disease is crucial
In 2023, controversy reigned over the FDA’s decision to approveand, due to a lack of evidence for their efficacy. One of the limitations of the trials of these drugs had been the inability to start their use earlier in the progression of the disease, potentially affecting results, it was argued at the time.
“As Alzheimer’s specialists, we very much want a blood test that confirms the diagnosis. This has become urgent in the last year, as there is now an FDA-approved drug for Alzheimer’s that we call a Disease Modifying Therapy (DMT). At the moment, we rely on either a PET scan or a Lumbar Puncture. Both are expensive and somewhat invasive tests.”
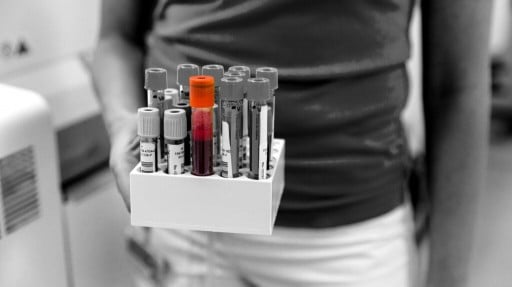
Can an Alzheimer’s blood test help early diagnosis?
There has been significant research into determining the form of tau found in the blood that is the most likely to indicate raised levels of tau and amyloid in the brain, and not elsewhere in the body. Previous research has looked at the ratio of certain forms of amyloid beta compared to other forms, and p-tau217 has also emerged as a form of tau that is indicative of the development of amyloid and tau buildup in the brain.
In 2023, a blood test for detecting amyloid beta protein was developed and made available by a company called AD-Detect. They also created the ALZpath pTau217 assay, which is only available to researchers but allows them to detect this form of tau in a blood test.
This was made available, free of charge, to an international group of researchers based across Scandinavia, Europe, and the United States to determine how this assay compared to using cerebrospinal fluid to biomarkers used to detect Alzheimer’s disease.
Detecting amyloid beta, tau proteins in the brain
Researchers looked at data from three sources — a cohort of individuals at risk of Alzheimer’s disease — and the other two were biobanks set up to investigate neurodegenerative conditions or aging and dementia. There were a total of 786 participants with a mean age of 66, including 504 female and 282 male participants.
Analysis of data showed that the assay was able to identify individuals in which there were abnormal levels of amyloid beta and tau but did not look at whether or not this correlated with the incidence of Alzheimer’s disease. Researchers tested the assay in conjunction with imaging data.
They found that the assay provided more accurate detection of hippocampal atrophy and was similar in its ability to detect abnormal levels of amyloid beta and tau as cerebrospinal fluid tests. This was the case across the three groups.
The authors said that the assay could detect abnormal levels of amyloid beta and tau in 80% of tested participants and that the remaining 20% would need confirmatory imaging or cerebrospinal fluid tests. They claimed this was more accurate than current diagnostic tools and could be used to identify people for early intervention with emerging pharmaceutical treatments designed to reduce amyloid buildup.
The makers of the assay reviewed the manuscript before it was submitted to the journal.
Clinical diagnosis of Alzheimer’s remains challenging
Dr. Clifford Segil, a neurologist at Providence Saint John’s Health Center in Santa Monica, CA, who was not involved in the research, told MNT that a blood test was not necessarily the answer for early detection. Dr. Segil said: “The authors noted there is no clear relationship to serum tau protein levels and cognitive complaints and therefore has no clinical utility for a neurologist or any physician to evaluate a patient with memory loss in 2024.”
“I strongly disagree, as a clinical neurologist who diagnoses and treats patients with cognitive complaints and memory loss, that blood biomarkers have any role in the clinical evaluation of patients with memory loss and dementia and would agree these may have a role in pharmaceutical drug trial enrollment as proposed by this paper.
[T]he goal to reduce Alzheimer’s diagnoses on advanced confirmatory tests is a pharmaceutical company goal and not a neurologist’s goal.”
– Dr. Segil
He warned there were risks with the approach proposed:
“If biomarkers are used millions of people will be diagnosed with Alzheimer’s Disease who may have no memory loss or cognitive impairment. A blood test for tau has no place to be used in a primary care physician’s office or a neurologist’s office in the clinical evaluation of patients with memory loss or cognitive impairment being evaluated for Alzheimer’s dementia. It will diagnose patients with Alzheimer’s dementia who are without memory loss and may never have memory loss.”